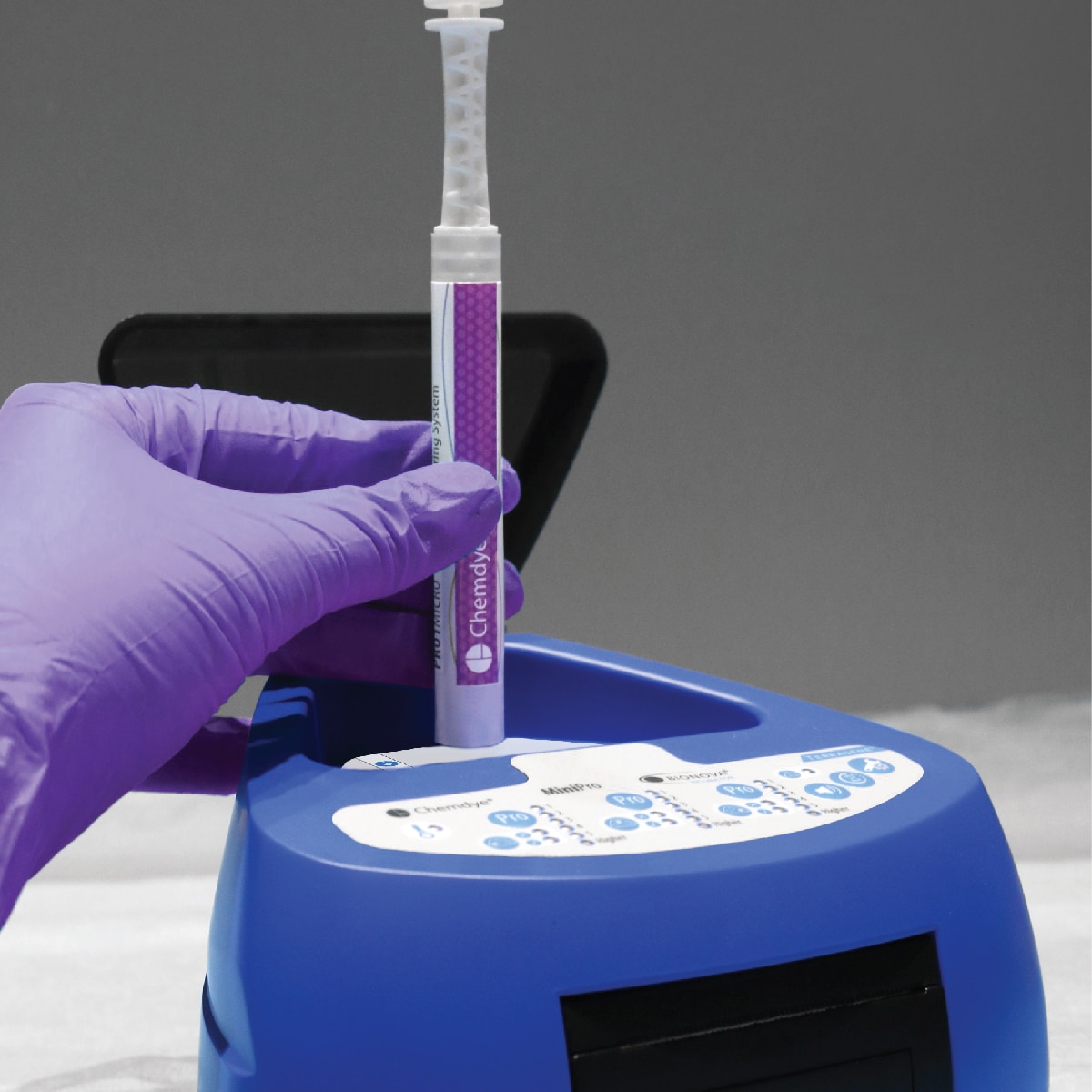
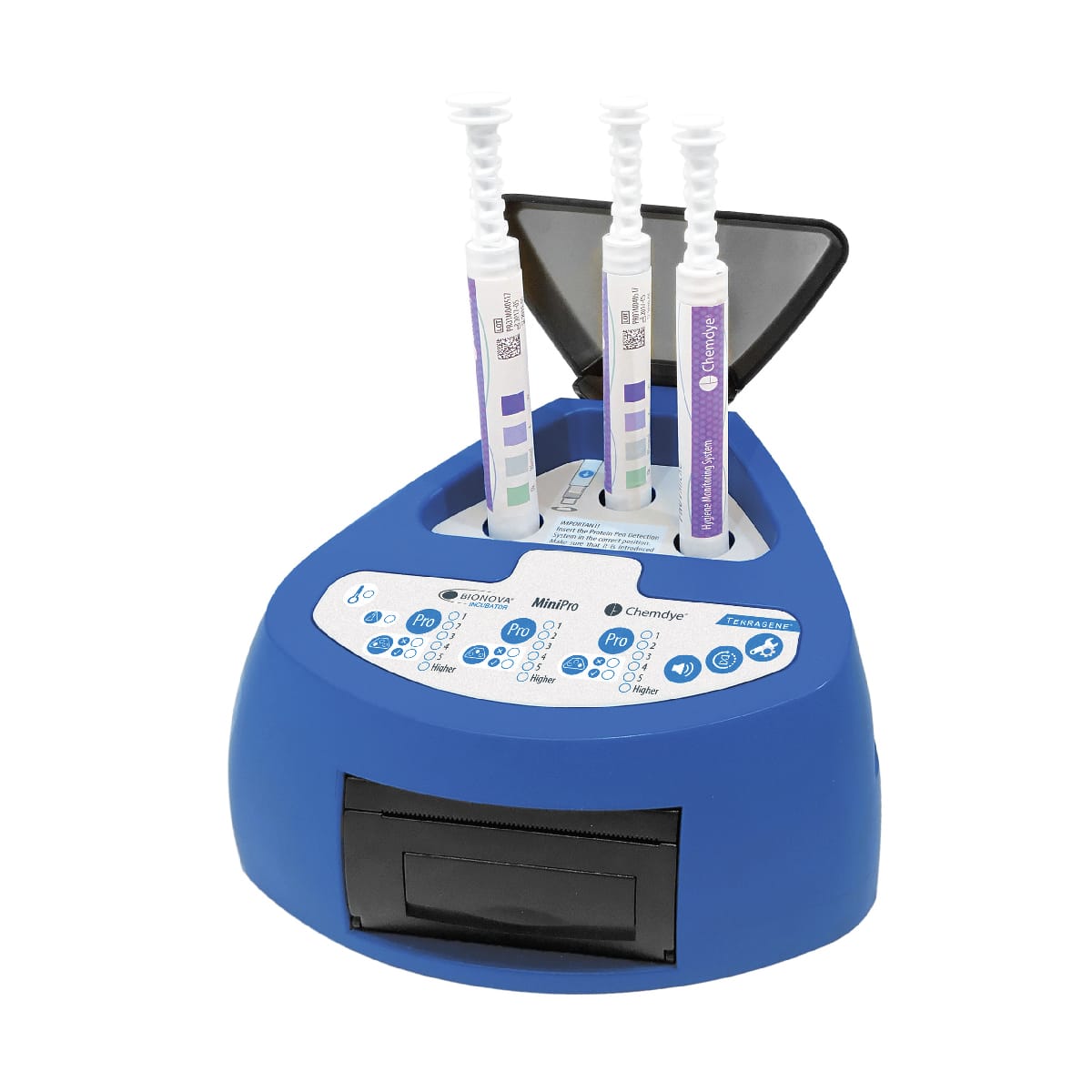
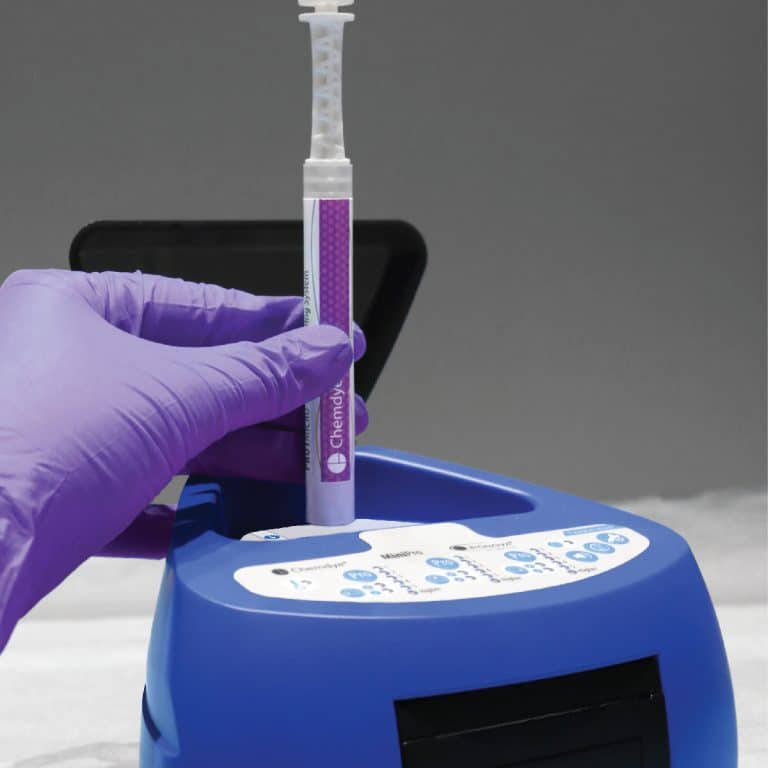
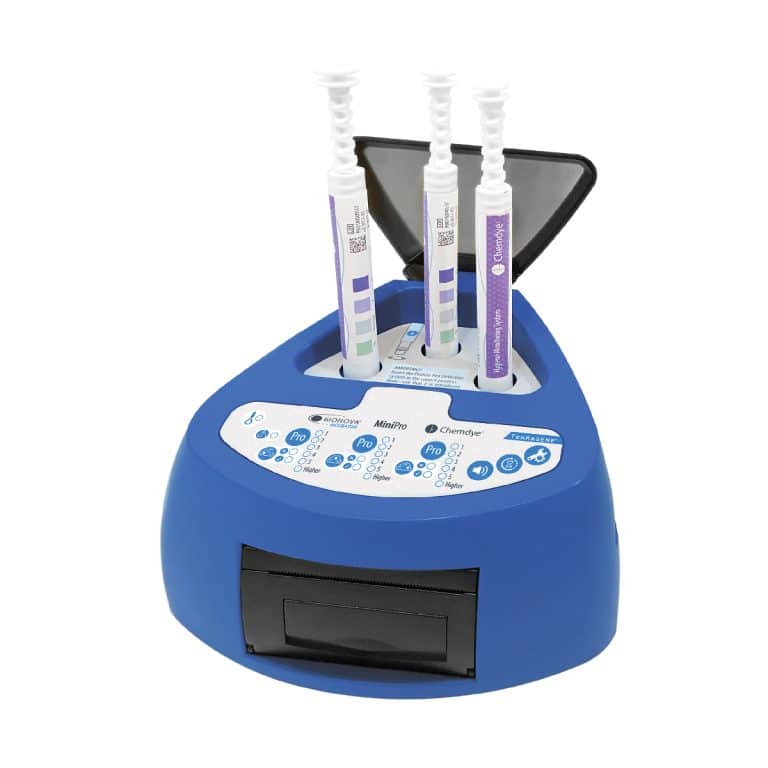
For incubation and quantitative readout of the pen system Chemdye® PRO1 MICRO
- 3 positions for simultaneous incubation and transmittance readout of hygiene indicators
- Results in 4 minutes
- Orifice for an external thermometer
- USB connection for recording results on PC using a Readout and Traceability software
- Thermal Printer results
- Sound alarm for indication of events
Possible target markets
Healthcare, Food, Pharmaceutical and medical devices industries
Designed under Quality Management System standards ISO 13485:2016/NS-EN ISO 13485:2016.
Low Voltage Directive 2014/35/EU.
Electromagnetic Compatibility Directive 2014/30/EU.
RoHS Directive 2011/65/EU.
WEEE Directive 2012/19/EU
Specification
Dimensions: 102 mm high, 156 mm wide and 146 mm deep
Voltage Range: 100 – 240 V AC.
Power: 28 W.
Frequency: 50-60 Hz.
To achieve full compliance with the latest HTM 01 – 01: 2016 for the decontamination of reusable surgical instruments, evidence of good practices is required, including logging evidence of each protein residue test carried out within the decontamination department as proof of a traceable process. Protein residue tests are essential to the day to day running of a sterile service department.
Discover more about Terragen’s MiniPRO on their Youtube Channel here.













Reviews
There are no reviews yet.