At Sychem, we are experts in all things Decontamination.
Supplying Infection Control consumables to countless SSD units across the UK, Sychem recognise the dire importance of the Decontamination cycle taking place as it should.
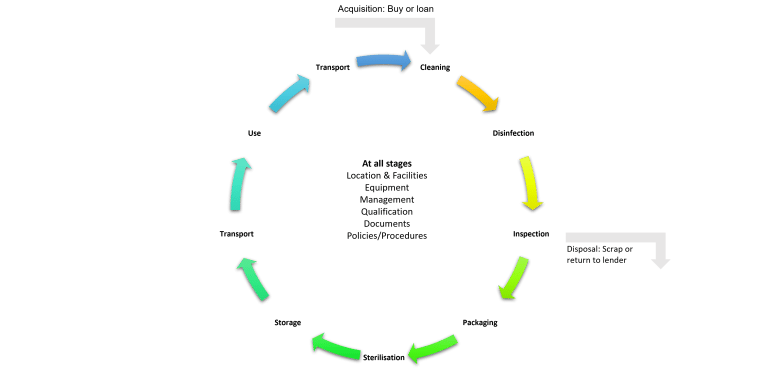
What is Decontamination?
Decontamination is a combination of processes that works to remove or destroy all forms of contamination.
The Decontamination process is an essential stage in device reprocessing as an instrument or device cannot be sterilised until it is completely clean.
In the decontamination process, soiled instruments are sorted, inspected and, if necessary, disassembled.
Instruments are first manually cleaned before any Capital equipment is used.
The Decontamination cycle?
The Decontamination cycle consists of multiple stages to ensure that the reprocessing of surgical instruments goes correctly. Some of the critical stages include:
- Pre-sterilisation cleaning
- Disinfection
- Inspection
- Sterilisation
- Storage
Pre-sterilisation cleaning
- The reasons CSSD staff may choose to manually clean a device/instrument include:
- The OEM recommends the device be manually cleaned.
- Hospital policy dictates the device must be manually cleaned and then processed in an automated washer/disinfector.
- Delicate instruments or powered equipment may not be suitable to be processed in an automated washer/disinfector.
- Manual cleaning requires either a two-bay sink or a three-bay sink. In a three-sink method, each bay plays a role in the cleaning process.1
- Sink 1: Instruments are immersed in an enzymatic solution to begin breaking down soils
- Sink 2: Instruments are immersed in a detergent solution and manually brushed
- Sink 3: Instruments are thoroughly rinsed with clean, treated water
- If a two-bay sink is being used, the process combines the enzymatic solution and detergent solution in one bay. The second bay contains clean, treated water as with the three-bay sink. If cleaning a lumened instrument or device, a brush or flushing with pressurised water may be used to loosen soils. Lubricant may be applied after manual cleaning.
- The reasons CSSD staff may choose to mechanically clean a device using an ultrasonic cleaner or irrigator and a washer/disinfector include:
- Washer/disinfectors offer increased productivity compared to staff manually cleaning.
- Washer/disinfectors provide a consistent, repeatable cleaning process so staff can be sure devices are thoroughly cleaned every time.
- One form of mechanical cleaning is ultrasonic cleaning. Ultrasonic cleaners clean instruments through acoustic cavitation, which forms air bubbles that implode on an instrument’s surface. These air bubbles can reach small crevices and hard-to-reach areas on a device. Ultrasonic cleaners are typically used to clean devices that may be sensitive to damage, and are too delicate for a traditional washer/disinfector. Ultrasonic cleaners have two chambers, and may come in a variety of sizes and types depending on the department’s need: freestanding, tabletop, large capacity, etc.
- The mechanical cleaning process may also be done via automated washer/disinfectors, which are available as single-chamber or multi-chamber. The washer/disinfector combines impingement, water temperature, and detergent to clean devices.
- In both ultrasonic cleaner and washer/disinfector processes, cleaning indicators are often used to monitor and evaluate the performance of the wash cycle.
Disinfection
• Washer/disinfectors offer increased productivity compared to staff manually cleaning
• Washer/disinfectors provide a consistent, repeatable cleaning process so staff can be sure devices are thoroughly cleaned every time
• One form of mechanical cleaning is ultrasonic cleaning. Ultrasonic cleaners clean instruments through acoustic cavitation, which forms air bubbles that implode on an instrument’s surface. These air bubbles can reach small crevices and hard-to-reach areas on a device. Ultrasonic cleaners are typically used to clean devices that may be sensitive to damage, and are too delicate for a traditional washer/disinfector. Ultrasonic cleaners have two chambers, and may come in a variety of sizes and types depending on the department’s need: freestanding, tabletop, large capacity, etc.
• The mechanical cleaning process may also be done via automated washer/disinfectors, which are available as single-chamber or multi-chamber. The washer/disinfector combines impingement, water temperature, and detergent to clean devices.
• In both ultrasonic cleaner and washer/disinfector processes, cleaning indicators are often used to monitor and evaluate the performance of the wash cycle.
Inspection and Packing
- After cleaning, instruments should be inspected for cleanliness and checked for damage before sterilisation. If instruments are damaged or still contaminated they should be rejected and re-cleaned.
- Once checked, the instruments are packed and wrapped, reading for sterilisation.
Sterilisation Process
- Once the instrument has been manually cleaned, mechanically cleaned, or both, it will be sent to the preparation and packaging area. Once the instrument pack has been prepped for sterilisation, it is ready to be sterilised through one of many methods of sterilisation.
- The primary method of medical instrument sterilisation include:
- Steam Sterilisation – Steam sterilisation is the predominant form of sterilisation. A steam steriliser, also known as an autoclave, is suitable for sterilising heat and moisture-stable items. Steam sterilisation cycle types include gravity, pre-vacuum and SFPP (Steam Flush Pressure Pulse). Cycle time varies according to cycle type, load weight and density and other variables such as exposure and drying time. At the end of the sterilisation cycle, the technician reviews the steriliser printout to verify if all sterilisation parameters have been met.
- Biological and chemical indicators are used to monitor the sterilisation process and indicate if the load was exposed to the appropriate conditions to achieve sterility.
Sterility Assurance
• Ensuring an instrument is sterile and safe to use is vital to reprocessing. Sterility assurance monitoring can be done through various forms of test packs, chosen based on the type of sterilisation process used or parameters being measured. A passing biological and chemical indicator test confirms that specific parameters of a sterilisation cycle were met. Some types of sterility assurance products include:1
• Biological Indicators – Biological indicators (BI) are designed to challenge the lethality of a sterilisation process to kill bacterial spores. Biological indicators are used within process challenge devices or challenge packs for routine monitoring, load monitoring and qualification of the sterilisation systems. The frequency for using biological indicators is based on the standards, the manufacturer’s instructions for use and the facility policies and procedures. A passing BI result indicates that the load can safely move on to sterile storage or the OR.
•Chemical Indicators – Chemical Indicators (CI) may be applied externally or internally to the package container. External chemical indicators, also called process indicators, show that the set has been fully exposed to the sterilisation process. Internal chemical indicators, which come in various forms, are placed in the most challenging area of the set and are read by OR staff to confirm that the sterilant penetrated the load.
•Bowie Dick Test – Bowie Dick Tests are required for steam sterilisers with a pre-vacuum cycle to check the efficiency of the air removal and steam penetration in the chamber. This test must be run daily before any pre-vacuum cycles are run.
Storage
• Sterilised instruments should be stored in clean, dry, covered conditions.
• Usually stored in a Sterile Store
Contact a member of our expert team to find out more.
Can’t quite find what you’re looking for? Visit the Terragene website for more information!